
TampoRelief 2/1
PROBLEM: 15 % of women experience severe period (menstruation) pain which seriously affects the quality of their lifes
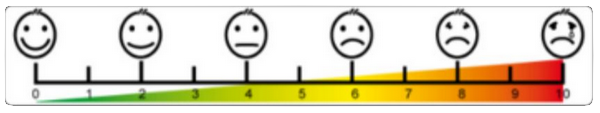
To evaluate pain in medicine, a visual analogue scale is used (scheme below): rate of 0 = no pain, 10 = unbearable pain. Our reasearch, performed on 240 Slovene woman not only confirmed, but even exceded the data of 15 % severe period pain from professional literature. It has shown that 13 % women evaluate their period pain with 7, 9 % with 8, 4 % with 9 and 1 % with 10. They confirm that first 2-3 days of their period they cannot be normally active, which severely affects the the quality of their lifes. It therefore accounts for 10 % of their reproductive age life.
CURRENT SOLUTIONS: hygenic product + painkiller (NSAID)
Current solution is not suitable for patients with gastrointestinal ulcers, hypersensitivity reactions, and those wishing to become pregnant. At the period, patient needs to have both products at hand, hygenic product and painkiller, which is not practical.
TampoRelief 2/1
TampoRelief 2/1 is a combination of hygenic product and medicine.. With 1 product 2 problems are solved. The onset of pain relief is substantially faster and due to an innovative use of known substance, no side effects exerted. The substrate for the introduction of the active pharmaceutical ingredient (API) is at the same time glidant, which facilitates the use of tampons and improve the user experience. Laboratory prototype is made of high quality, 100 % cotton and belongs to a group with a mean absorbance: 9-12 g of liquid. API is dissolved in the substrate of coloured PEG at the tip of the tampon.
At the picture, laboratory prototype is shown with API in coloured PEG before (A) and after absorbtion of the water (B). Water simulates body fluids. It is important that PEG stays at the tampon after in contact with water or body fluids.
The industrial prototype, suitable for clinical testing, will be prepared in 1 form. After commercialisation, product extensions are planned, suitable for different needs of patients: